PATHOPHYSIOLOGY
In 1958, Scheie and Fleischauer (95) described iris transillumination defects associated with
PDS and attributed them to IPE atrophy. With no real evidence except a "somewhat waxy
or pale" appearance of the ciliary body in a few patients, they extended the
hypothesis of congenital atrophy to include this structure.
Fine, Yanoff and Scheie (33) examined the eyes of a 55 year-old man who had been
found to have PDS without glaucoma at age 43. In the iris midperiphery, there was an
abrupt transition from normal to abnormal IPE. In this region, IPE loss was accompanied by
hyperplasia of the iris dilator muscle and there was marked hyperplasia of the muscle spur
of Grunert at the iris root. Pigment epithelial cells appeared to be migrating anteriorly
and differentiating into smooth-muscle-containing cells. Rodrigues et al, (91) on the other hand, reported a focally thickened dilator
muscle with thinning in the areas of epithelial atrophy. They found an increased number of
immature melanosomes in the IPE and suggested that of a delay in melanogenesis occurred as
part of a developmental defect.
Kupfer et al (57)
considered the primary lesion in PDS to be an epithelial abnormality. The dilator fibers
of the inner IPE appeared to be hypertrophic and hyperplastic, resembling the sphincter
muscle, and were associated with degenerated neural elements. They felt that the primary
defect lay in the inner IPE and could represent a congenital or developmental abnormality,
but could also be the result of interruption of sympathetic innervation.
The relevance of dilator muscle hyperplasia
and nerve fiber degeneration to the disease process remain unknown. The possibility of an
adrenergic hypersensitivity in patients with PDS and PG might explain comments made in
passing that epinephrine compounds, alone or in combination with other agents, seem to be
more effective in patients with PG than in those with POAG. (7,94)
Campbell (18,19) proposed that posterior bowing of the iris brings it
into contact with the anterior zonular bundles. The location and number of the
transillumination defects correlated with the position and number of the underlying
zonular bundles. He noted that hyperplasia of the iris dilator muscle was localized to
areas of iridozonular contact and hypothesized that iridozonular friction during pupillary
movement disrupts the IPE, releasing pigment into the posterior chamber. Scanning electron
microscopic observations supported this hypothesis. (22,48)
Back
ULTRASOUND
BIOMICROSCOPIC FINDINGS
The advent of high frequency, high
resolution, anterior segment ultrasound biomicroscopy has enabled us to elucidate a number
of facets of the pathophysiology of PDS. (39,63-65,79,80,83,86,99) One overall impression obtained from imaging studies is
that the size of the iris is overly large relative to that of the anterior segment
(figures 4-6). This may be the basic anatomic cause of the midperipheral iris concavity
and predispose to iridozonular contact. Sokol et al (99)
compared patients with PDS to age-, sex-, and refraction-matched controls and found a
greater mean iris-trabecular meshwork distance in the PDS group. Thus, iridozonular
contact appears to be facilitated by a congenitally more posterior iris insertion.
Both iridozonular and iridociliary contact
have been imaged. Although iridociliary contact does not appear to be much of a factor in
pigment liberation, the occasional extension of transillumination defects into the
periphery of the iris, creating an appearance similar to that of an exclamation point,
suggests that contact between the two surfaces may damage the pigment epithelium of both
and may account retrospectively for the observation of Scheie and Fleischauer regarding
the "pale and waxy" appearance of some ciliary processes.
Back
BLINKING
Lid blinking may be important in determining
iris configuration. Campbell (21) noted and Liebmann et
al (64) confirmed that when blinking is prevented in PDS
patients, aqueous humor builds up in the posterior chamber and the iris assumes a planar
and even a convex configuration. As the volume of the posterior chamber increases relative
to that of the anterior chamber, the iris gradually flattens, iridolenticular contact
diminishes, and iridozonular and iridociliary process distances increase. In the most
pronounced cases, iridolenticular contact disappears, the iris sphincter lifting
completely off the surface of the lens without the posterior chamber losing its expanded
volume (figure 7). Eyes with PDS take longer to reach a steady-state position because
their initial iris concavity is greater than that of control eyes. (64)
The mechanism by which blinking affects the
anatomy of the anterior segment appears to be a mechanical one. Campbell (21) proposed that a blink initially deforms the cornea,
transiently increasing IOP and pushing the iris posteriorly against the lens. When PDS
patients are permitted to blink and rescanned, the concave iris configuration returns in
all eyes. (64) Chew et al (24)
demonstrated that during blinking of the nictitating membrane in the chick eye, the cornea
indents in a wave from the periphery to the center and that anterior chamber depth
similarly decreases (figures 8 and 9).
Extrapolating this to humans, we hypothesize
that blinking acts as a mechanical pump to push aliquots of aqueous humor from the
posterior chamber to the anterior chamber. A pressure wave is created, pushing the iris
posteriorly toward the zonules. This wave begins at the iris periphery and moves
centrally, pushing aqueous before it into the anterior chamber and emptying the posterior
chamber. Abnormally extensive iridolenticular contact in eyes with PDS prevents
equilibration of aqueous between the anterior and posterior chambers, a situation which
has been termed reverse pupillary block. (21,49) At the same time, the iris reassumes its concave
configuration. The now increased volume of aqueous in the anterior chamber helps to
maintain the midperipheral iris concavity, although whether or not there is a pressure
gradient accentuating the concavity remains to be shown. As aqueous leaves the eye through
the meshwork and enters via ciliary secretion, the anterior chamber volume decreases and
the posterior chamber volume increases, until the next blink starts the cycle all over
again. Interestingly, increasing myopia is also a predictor of increasing iridolenticular
contact, independent of the presence of PDS. (64) This
may explain why myopia enhances the phenotypic expression of the genetic abnormality
underlying PDS. It also raises the question as to whether decreased trabecular function
and reduction of the aqueous outflow coefficient might serve to accentuate the iris
concavity.
Back
Figure 8.
Concave iris posterior to the reference line at the time of initial scanning (Time 0).
Iridolenticular contact is present and the iris has a concave configuration. |
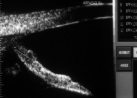 |
Figure 9.
During continuous scanning, the patient is not able to blink because of the eye cup which
has been inserted between the lids. Three minutes after the initiation of scanning,
aqueous production within the posterior chamber has caused the iris to move anteriorly and
become less concave. |
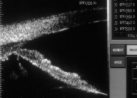 |
Figure 10.
The iris has become less concave 7 minutes after the initiation of scanning. Note the
position of the reference line to the iris pigment epithelium. |
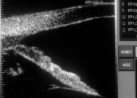 |
Figure 11.
The maximal change in iris configuration occurred at 11 minutes. The iris is now convex
and anterior to the reference line. There is no iridolenticular contact. |
 |
Figure 12.
The iris configuration remained unchanged for the next 11 minutes. At 22 minutes, the iris
configuration was nearly identical to the configuration at 11 minutes. During this time,
aqueous was produced in the posterior chamber, is able to move into the anterior chamber
(via the pupillary space), and exit the eye via the trabecular meshwork. |
 |
Figure 13.
The iris configuration remained unchanged for the next 11 minutes. Following removal of
the eye cup at 23 minutes, the patient was instructed to blink. The iris is now concave,
although less so than at the time of initial scanning. Iridolenticular contact is present. |
 |
ACCOMMODATION
Accommodation in normal, young individuals
and PDS patients may also affect iris contour. (64,73) Accommodation in normal eyes causes an iris concavity
indistinguishable from that in PDS. Contraction of the ciliary ring allows shallowing of
the anterior chamber, anterior lens movement, and increased iridolenticular contact.
Aqueous in the anterior chamber is forced into the angle recess and the peripheral iris
becomes more concave. As accommodation is relaxed, the iris resumes its initial
configuration.
Accommodation might enhance pigment
liberation in two ways. In addition to posterior iris bowing during accommodation, the
pupil constricts. Relaxation of accommodation accompanied by pupillary dilation might
result in additional iridozonular friction. Ultrasound biomicroscopy during accommodation
in eyes with PDS shows iridozonular contact at the lens margin, consistent with the usual
position of iris transillumination defects. (81)
Back
Figure 14.
Iris position upon initial scanning in a normal, emmetropic, 20 year-old man fixing on a
wall-mounted target. The iris has a minimally convex configuration. |
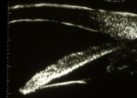 |
Figure 15.
As soon as fixation is moved to a near target (30 cm), contraction of the ciliary
muscle causes anterior movement of the lens, increasing iridolenticular contact, which
effectively traps aqueous within the anterior chamber. Because of the anterior chamber
shallowing, aqueous is forced peripherally, and gives the iris a concave configuration.
The ciliary body appears to be indenting the peripheral iris. |
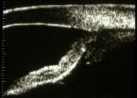 |
Figure 16.
Upon relaxation of accommodation and fixation on the distant target, the iris resumes its
prior convex configuration. |
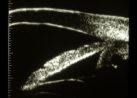 |
Figure 17.
Iris position upon initial scanning in a 27 year-old, myopic (-0.50 D) man with pigment
dispersion syndrome fixing on a wall-mounted target. The iris has a minimally concave
configuration. |
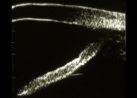 |
Figure 18.
As soon as fixation is moved to a near target (30 cm), contraction of the ciliary muscle
causes anterior movement of the lens, increasing iridolenticular contact, which
effectively traps aqueous within the anterior chamber. Because of the anterior chamber
shallowing, aqueous is forced peripherally, and gives the iris a concave configuration. |
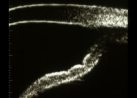 |
Figure 19.
Upon relaxation of accommodation and fixation on the distant target, the iris resumes
its prior concave configuration. |
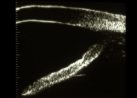 |
EFFECT OF
MIOTICS AND LASER IRIDOTOMY
Scanning following administration of
pilocarpine shows resolution of the iris concavity and iridozonular contact in all eyes.
Pilocarpine produces a convex rather than a planar configuration. Laser iridotomy relieves
reverse pupillary block by allowing aqueous to flow from the anterior to the posterior
chamber and produces a planar iris configuration (figures 5 and 6). Some eyes undergoing
iridotomy may still retain a concave iris configuration.(45)
Iridotomy does appear to prevent the accentuation of the iris concavity which accompanies
accommodation. (81) Some PDS patients may develop IOP
rises after shedding pigment with exercise or with pupillary dilation. (29,37,55,69,82,96,109) The exercise-induced release of pigment and elevation
of IOP can be blocked by pilocarpine. (20,41,96) Whereas pilocarpine
completely inhibits exercise-induced pigment release and IOP elevation, iridotomy does so
incompletely. (39,41)